Research Interests
We are a structural biology lab that is interested in method development and in understanding how ion channels, and the macromolecular complexes that regulate them, function in health and disease. Primarily, we will be applying cryo-electron microscopy (cryo-EM) and cryo-electron tomography (cryo-ET) to reconstruct the biochemical and molecular processes underlying these problems. We firmly believe that the most informative mechanistic insights into biology come from a deep understanding of the biological machines that govern them.
Time-Resolved Cryo-EM
We are interested in developing time-resolved cryo-EM and cryo-ET to understand the atomistic details of how proteins work as a function of time. Despite the power of cryo-EM to elucidate the structural details of proteins and protein complexes, the temporal resolution of traditional specimen preparation is orders of magnitude slower than the pace at which life occurs. We are building cryo-EM specimen preparation devices that will allow us, and others, to capture biological processes at a physiologically relevant timescale.
Glutamate Receptors
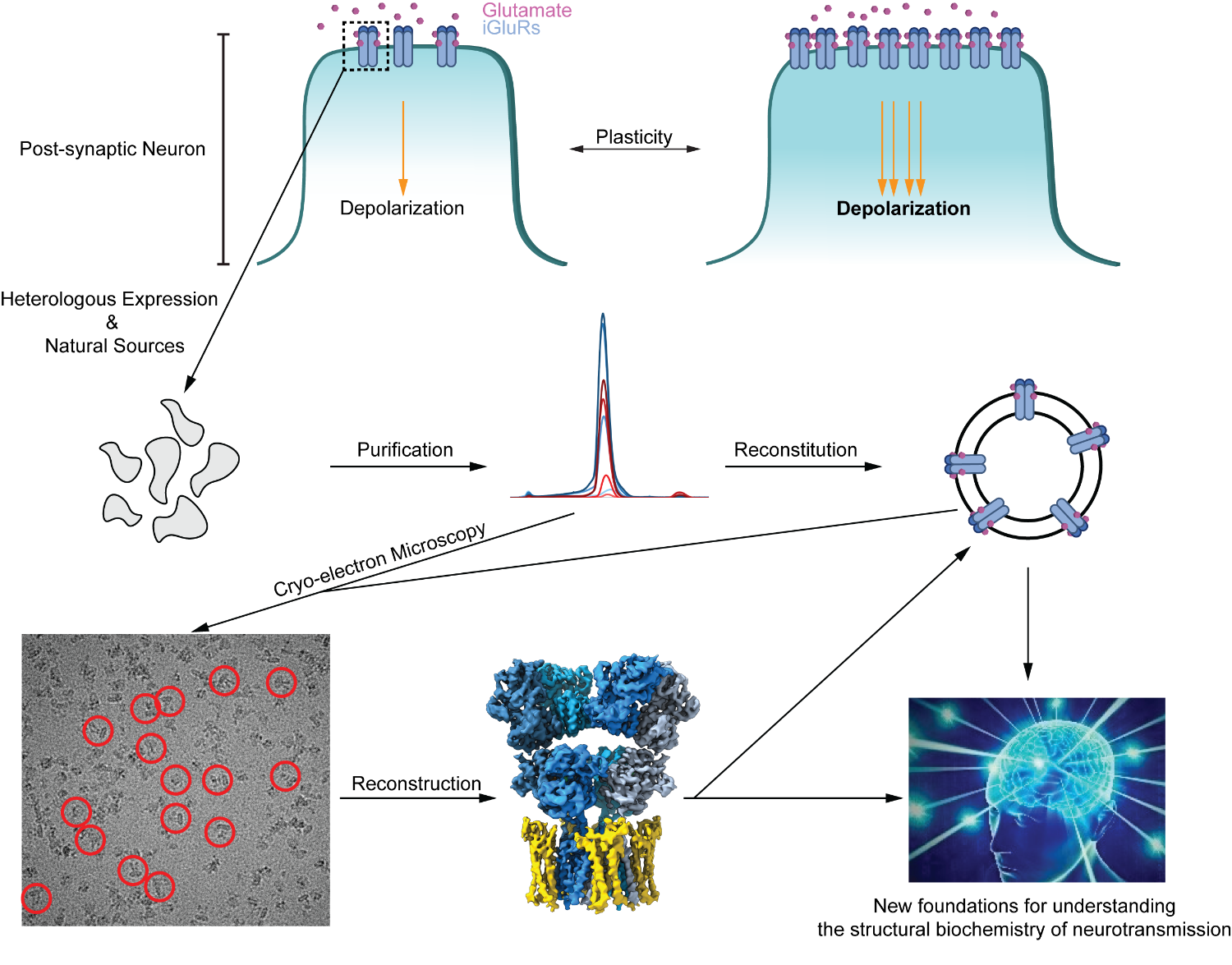
Most synapses (~90%) in the central nervous system (CNS) are glutamatergic. Here, ionotropic glutamate receptors (iGluRs) in the post-synaptic membrane bind glutamate released from pre-synpatic neurons, which triggers cation influx into the post-synaptic neuron and depolarization. A subset of these iGluRs, α-amino-3-hydroxy-5-methyl-4-isoaxazolepropionic acid (AMPA) receptors, dictate fast excitatory neurotransmission and thus the initial depolarization of the post-synaptic neuron. AMPA receptor complexes at the post-synaptic membrane, and the dynamics of their recruitment to, and removal from it, have profound effects on synaptic plasticity – the process by which patterns of synaptic activity result in a strengthening or weakening of transmission. The altered neurotransmission affects high cognitive processes such as learning and memory, and its dysregulation leads to neurodegeneration. A wealth of processes regulates AMPA receptors, from assembly in the endoplasmic reticulum to their function within the post-synaptic membrane, and their eventual endocytosis and degradation. We aim to reconstitute these processes in vitro to characterize them biochemically, and to use cryo-EM and structural biology techniques to understand structure-function relationships. We expect for these studies to not only provide new insights into the basic mechanisms of neurotransmission and insights into neurodegeneration, but also lay new foundations for rational drug design and understanding patient mutations.
Tight Junctions
Across all epithelial and endothelial tissues, tight junctions are paracellular gatekeepers that regulate tissue homeostasis and cell polarity as the most-apical cell-cell complex. Dysregulation of tight junctions can lead to cancers, neurodegeneration, visual impairment, gastrointestinal distress, and breathing impairment. Dysregulation or hijacking of tight junction components also plays a major role in viral entry, such as with Hepatitis C, Zika, and SARS-CoV-2. In addition, understanding tight junctions at the blood brain barrier is critical for improving CNS drug delivery. Despite the critical role that tight junctions play in physiology and disease, we lack a precise molecular and atomic understanding of how they function, how they assemble, as well as their architecture. This is a fundamental barrier in understanding the major barrier forming complex in tissues, and in drug design. We are using molecular modeling, cryo-EM, cryo-ET, single molecule biophysics, and cell biology to understand tight junctions.